Cancer can be caused solely by protein imbalances within cells, a study of ovarian cancer has found.
The discovery is a major breakthrough because, until now, genetic aberrations have been seen as the main cause of almost all cancer.
The research, published today in the journal Oncogene, demonstrates that protein imbalance is a powerful prognostic tool, indicating whether or not patients are likely to respond to chemotherapy and whether a tumour is likely to spread to other sites.
The findings also open the possibility of new therapies aimed at measuring and preventing dangerous imbalances in cells.
Lead author Professor John Ladbury, Dean of the University of Leeds’ Faculty of Biological Sciences and Professor of Mechanistic Biology, said: “There has been huge investment in sequencing the human genome with the idea that if we get all the relevant genetic information we can predict whether you have a predisposition to cancer and, ultimately, use a precision medicine-based approach to develop a therapeutic approach. Our study demonstrates that genetic screening alone is not enough.”
The research, led by scientists at the University of Leeds and The University of Texas MD Anderson Cancer Center, focused on the “Akt pathway,” a signalling pathway within cells that drives cancer formation and the spread of cancers through the body.
Under normal conditions, the cell receives external signals through a cell wall-bound receptor (FGFR2 in this study). As a result of this stimulus the receptor is ‘switched on’ inside the cell. This results in the recruitment of signalling proteins and the initiation of the Akt pathway, which is responsible for committing the cell to proliferate. In some cancerous cells, this pathway is permanently switched on. A conventional approach to diagnosing this cancer would be to look for genetic modification of the receptor (or recruited proteins), which could be responsible for maintaining the switched on state."
The new study looked at isolated cancer cells without external stimulation and found that the “Akt pathway” could be activated without genetic modifications. Two proteins; Plc?1 (pronounced “plc-gamma-1”) and Grb2 (pronounced “grab-2”), compete for binding to FGFR2. The relative concentration of these proteins will dictate which one binds. When Plc?1 prevails, it triggers the Akt pathway. In this way, an imbalance in the amount of the two proteins can lead to cell proliferation and cancer formation.
Dr Zahra Timsah, University Academic Fellow at the University of Leeds’ School of Molecular and Cellular Biology, who was the lead researcher on the study, said: “This competition for binding to the receptor represents an unexpected way in which cancer can occur. We found that in cells where Grb2 is depleted, FGFR2 was vulnerable to Plc?1 binding and that this triggered uncontrolled proliferation. Increasing the amount of Grb2 rescued this effect to maintain normal FGFR2 activity. What we think is happening is that under normal conditions the two proteins compete fairly evenly and that the Plc?1 binding events allow useful cell housekeeping. When the proteins get imbalanced, Plc?1 can get out of control.”
The researchers also looked at the process in a mouse model and found that Grb2 depletion results in the development of multiple tumours in the vicinity of a primary tumour, indicating that protein imbalance can have a role in metastasis, the spread of a cancer through the body. This makes sense because Plc?1 can play a role in increasing cell movement.
Finally, the researchers looked at whether imbalance between Grb2 and Plc?1 was predictive of the progress of ovarian cancers in patients. Measuring the levels of the proteins in patient tissues followed by database analysis of clinical information from The Cancer Genome Atlas and other sources revealed that a high level of Grb2 relative to Plc?1 and FGFR2 was associated with a significantly more favourable prognosis than patients with elevated levels of Plc?1.
Statistical data reveal that just under 40% of patients with a favourable balance were still alive seven years after samples were taken. Less than 10% of patients with high levels of Plc?1 and FGFR2 binding sites survived the same length of time.
Professor Ladbury said: “From the patient’s point of view, the key findings are that these proteins are biomarkers. They could offer information to clinicians on who is going to benefit from therapy and, just as importantly, who is not. On the treatment side, the proteins’ interaction could be a valid therapeutic target: you could, for instance, target Plc?1 to ensure it does not overwhelm the cell.”
Previous research findings have emphasised the roots of cancer in genetic mutation. Some studies have pointed to cancers that occur without genetic causes, such as through epigenetic modifications of proteins, however the present study reveals that signalling though cell wall-based receptors can occur without receptor activation and therefore that non-genetic causes may be critical to understanding cancer in large numbers of patients.
The researchers are now working with clinicians at the University of Leeds to study the same mechanisms in other forms of cancer. They are also exploring the possibility that other cell receptors could play a similar role to FGFR2 in sustaining oncogenic signalling without being activated themselves.
The research was funded by the G. Harold and Leila Y. Mathers Charitable Foundation, the National Institute of Health (NIH), the RGK Foundation and the Gilder Foundation. It involved researchers from the University of Leeds, The University of Texas MD Anderson Cancer Center and the UT Health Science Center at Houston.
Image information:
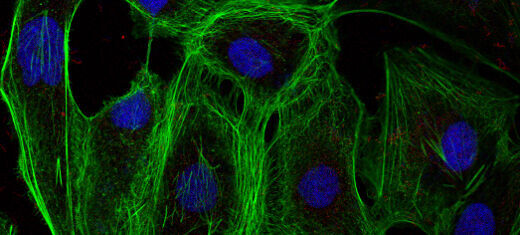
A group of ovarian cancer cells expressing low levels of the protein Grb2, making them vulnerable to cancerous proliferation. The cell nuclei are blue and the green colour shows filaments that facilitate the cancer’s mobility.
Further information:
Contact: Chris Bunting, Senior Press Officer, University of Leeds; phone +44 (0)113 343 2049 or email c.j.bunting@leeds.ac.uk.
The full paper: Z. Timsah et al., ‘Grb2 depletion under non-stimulated conditions inhibits PTEN, promotes Akt-induced tumor formation and contributes to poor prognosis in ovarian cancer’ is published in Oncogene (2015), 1-11 (DOI:10.1038/onc.2015.279; URL: http://www.nature.com/onc/journal/vaop/ncurrent/full/onc2015279a.html)