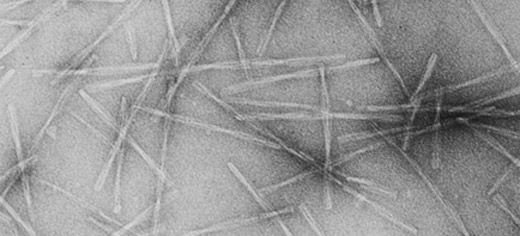
A molecule which can stop the formation of long protein strands, known as amyloid fibrils, that cause joint pain in kidney dialysis patients has been identified by Leeds researchers.
The discovery could lead to new methods to identify drugs to prevent, treat or halt the progression of other conditions in which amyloid fibrils play a part, including Alzheimer's, Parkinson's and Type II diabetes. The research, funded by the Biotechnology and Biological Sciences Research Council and the Wellcome Trust, is published today (August 28) in Nature Chemical Biology.
The team - from Leeds' Astbury Centre for Structural Molecular Biology and Faculty of Biological Sciences - found that an antibiotic known as Rifamycin SV was able to prevent the protein íŸ2microglobulin (íŸ2m) from forming into fibrils. íŸ2m is known to accumulate in renal dialysis patients and forms fibrils within the joints, causing extreme pain and arthritis.
By using a specialised analytical technique called ion mobility spectrometry-mass spectrometry (IMS-MS), the researchers were able to see at what stage of the process Rifamycin SV prevented amyloid fibril formation. They believe the technique could enable potential drugs to be identified for the many other proteins which form amyloid fibrils, linked to a wide range of human disorders.
"Traditional drug design for diseases like Alzheimer's is incredibly difficult because the proteins you're trying to target are changing shape and structure all the time," explains University of Leeds Professor of Structural Molecular Biology, Sheena Radford.
"It's like trying to consistently pick out one bead of a particular shape from box of potentially millions of similar beads. This new technique allows us to see the shape of the protein as it changes, so we can more easily identify exactly which part we need to target."
In their normal, folded state, proteins are unable to link together to form long fibrillar assemblies, but if they unfold, they expose areas where they can bind to each other. Initially they form small groups of two, three or four proteins, and then these link into long strands, which twist together to form fibrils.
Most analytical techniques can only show the mass of the protein or its make-up in terms of amino acids, neither of which changes as the protein unfolds. Others are unable to look at individual molecules within complex mixtures.
However, IMS-MS can measure the mass and shape of a protein, allowing researchers to watch the unfolding process and the aggregation into small groups and then assembly into the fibril and to find which of these species is able to bind a ligand and stop the assembly process.
In the research published today, researchers found that Rifamycin SV stopped the formation of protein fibrils by binding to an unfolded protein molecule with a particular shape, enabling for the first time, an unfolded protein of a particular shape to be identified as a target for the design of new inhibitors of fibril assembly.
"We're fortunate to be one of the few universities in the UK able to use IMS-MS to study amyloid fibril formation," says Professor of Biomolecular Mass Spectrometry, Alison Ashcroft, who specialises in this type of analysis. "Although fibrils take years to develop in the body, we are able to 'grow' them in hours in the lab.
By using IMS-MS to help us map exactly how they are formed, we can better understand the mechanism by which it happens and - we hope - find ways to stop it."
For further information:
Please contact the University of Leeds Press Office on +44 (0)113 343 4031 or email pressoffice@leeds.ac.uk
Notes to editors:
- The paper: Ligand binding to distinct states diverts aggregation of an amyloid-forming protein is published on August 28 via Advance Online Publication (AOP) on the Nature Chemical Biology website. DOI: 10.1038/NChemBio.635
- The papers authors are: Sheena Radford, Alison Ashcroft, Steve Homans, Eric Hewittt, Andrew Hellewell, Geoffrey Platt and Lucy Woods, of the Institute of Molecular and Cellular Biology, Faculty of Biological Sciences at the University of Leeds.
- The Faculty of Biological Sciences at the University of Leeds is one of the largest in the UK, with over 150 academic staff and over 400 postdoctoral fellows and postgraduate students. The Faculty is ranked 4th in the UK (Nature Journal, 457 (2009) doi :10.1038/457013a) based on results of the 2008 Research Assessment Exercise (RAE). The RAE feedback noted that "virtually all outputs were assessed as being recognized internationally, with many (60%) being internationally excellent or world-leading" in quality. The Faculty's research grant portfolio totals some £60M and funders include charities, research councils, the European Union and industry. http://www.fbs.leeds.ac.uk/
- The 2008 Research Assessment Exercise showed the University of Leeds to be the UK's eighth biggest research powerhouse. The University is one of the largest higher education institutions in the UK and a member of the Russell Group of research-intensive universities. The University's vision is to secure a place among the world's top 50 by 2015. http://www.leeds.ac.uk/
- Biotechnology and Biological Sciences Research Council (BBSRC) is the UK funding agency for research in the life sciences and the largest single public funder of agriculture and food-related research.
Sponsored by Government, in 2010/11 BBSRC is investing around £470 million in a wide range of research that makes a significant contribution to the quality of life in the UK and beyond and supports a number of important industrial stakeholders, including the agriculture, food, chemical, healthcare and pharmaceutical sectors. BBSRC provides institute strategic research grants to the following: The Babraham Institute, Institute for Animal Health, Institute for Biological, Environmental and Rural Studies (Aberystwyth University), Institute of Food Research, John Innes Centre, The Genome Analysis Centre, The Roslin Institute (University of Edinburgh) and Rothamsted Research. The Institutes conduct long-term, mission-oriented research using specialist facilities. They have strong interactions with industry, Government departments and other end-users of their research.
For more information see: http://www.bbsrc.ac.uk/ - The Wellcome Trust is a global charitable foundation dedicated to achieving extraordinary improvements in human and animal health. It supports the brightest minds in biomedical research and the medical humanities. The Trust's breadth of support includes public engagement, education and the application of research to improve health. It is independent of both political and commercial interests. http://www.wellcome.ac.uk/