Scientists have shown that gold nanotubes have many applications in fighting cancer: internal nanoprobes for high-resolution imaging; drug delivery vehicles; and agents for destroying cancer cells.
Gold nanotubes – that is, gold nanoparticles with tubular structures that resemble tiny drinking straws – have the potential to enhance the efficacy of these conventional treatments by integrating diagnosis and therapy in one single system.”
The researchers say that a new technique to control the length of nanotubes underpins the research. By controlling the length, the researchers were able to produce gold nanotubes with the right dimensions to absorb a type of light called ‘near infrared’.
The study’s corresponding author Professor Steve Evans, from the School of Physics and Astronomy at the University of Leeds, said: “Human tissue is transparent for certain frequencies of light – in the red/infrared region. This is why parts of your hand appear red when a torch is shone through it.
“When the gold nanotubes travel through the body, if light of the right frequency is shone on them they absorb the light. This light energy is converted to heat, rather like the warmth generated by the Sun on skin. Using a pulsed laser beam, we were able to rapidly raise the temperature in the vicinity of the nanotubes so that it was high enough to destroy cancer cells.”
In cell-based studies, by adjusting the brightness of the laser pulse, the researchers say they were able to control whether the gold nanotubes were in cancer-destruction mode, or ready to image tumours.
In order to see the gold nanotubes in the body, the researchers used a new type of imaging technique called ‘multispectral optoacoustic tomography’ (MSOT) to detect the gold nanotubes in mice, in which gold nanotubes had been injected intravenously. It is the first biomedical application of gold nanotubes within a living organism. It was also shown that gold nanotubes were excreted from the body and therefore are unlikely to cause problems in terms of toxicity, an important consideration when developing nanoparticles for clinical use.
“The nanotubes can be tumour-targeted and have a central ‘hollow’ core that can be loaded with a therapeutic payload. This combination of targeting and localised release of a therapeutic agent could, in this age of personalised medicine, be used to identify and treat cancer with minimal toxicity to patients.”
The use of gold nanotubes in imaging and other biomedical applications is currently progressing through trial stages towards early clinical studies.
This work was supported by the Wellcome Trust, with additional funding provided by the Engineering and Physical Sciences Research Council (EPSRC).
Dr Sunjie Ye, Professor Steve Evans and Dr James McLaughlan are available for interview. Please contact Sarah Reed, Press Officer, University of Leeds, on 0113 34 34196 or email s.j.reed@leeds.ac.uk
Image download: http://goo.gl/IhNt4i
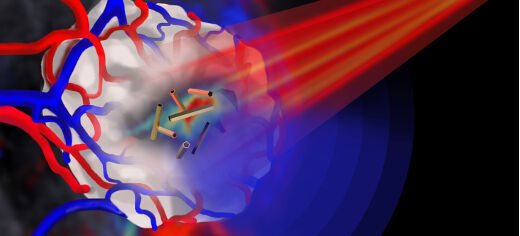
Image caption: Pulsed near infrared light (shown in red) is shone onto a tumour (shown in white) that is encased in blood vessels. The tumour is imaged by multispectral optoacoustic tomography via the ultrasound emission (shown in blue) from the gold nanotubes.
Image credit: Jing Claussen (iThera Medical, Germany)